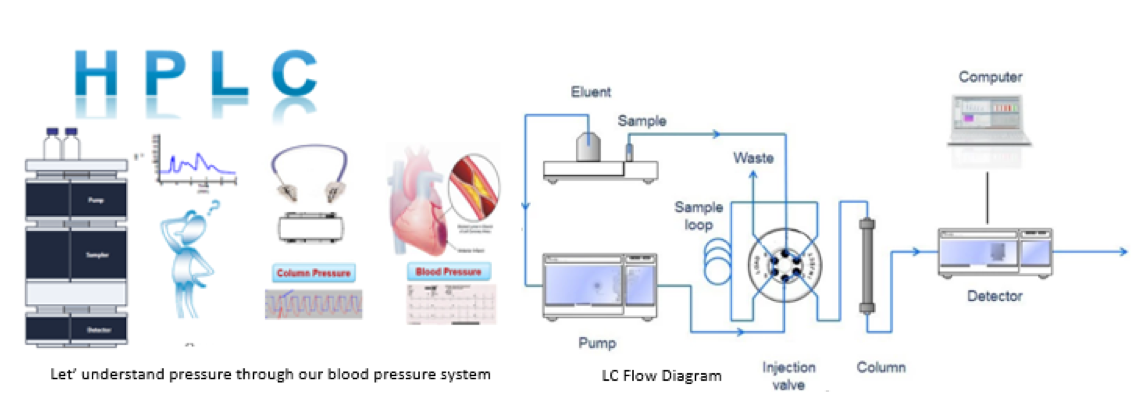
The Typical High Pressure liquid chromatography system configuration contains Solvent cabinet, degasser, pump, autosampler, column compartment and detector. HPLC is a basic detector which help to separate the compounds and detects. The deferent detectors are VWD, DAD,MWD, RID, FLD, ELSD etc and helps for qual and quant analysis. HPLC system are used as front end for sample introduction for LCMS detectors worldwide in the field of liquid chromatography.
Common problems are Pressure fluctuation, RT variation, peak tailing, peak broadening, split peaks, carryover, base line drift etc. It’s very important to know the basics of troubleshooting and healthcare of the instrument. The common instrument care.
- Use Clean solvent inlet glass filter must be in use.
- Reservoir must be covered with aluminum foil for critical mobile phase to avoid evaporation.
- Solvent channel must be purged in case of solvent change or when system is not in use for long time.
- The vacuum degasser can be primed with syringe when channel has dried out.
- Mobile phase must be filtered through proper filter.
- To avoid long storage of buffer (4-8pH) or water as it promotes the algae formation.
- Aqueous phase must be stored in amber color bottle.
- Seal wash with 10% IPA in water keeps healthy piston and seals.
- System need to flush with water for salt removal and periodically thorough washing with warm water > IPA : water > methanol
- Column must be replaced with union/dead volume during flushing.
- Inline filters need to be changed at set interval.
- Use automatic or wash vial option for needle wash to avoid carry over.
- Use appropriate solvent for needle wash.
- Do not use the cap on wash vial.
- If available use injector program to reduce carryover.
- Periodically wipe the needle seat with water : IPA.
- Use recommended vials and septum.
- Do not over fill the vial.
- Filter the sample solution through proper filter.
- Gentle operation required for fixing the capillary.
- Give detector’s optical unit proper time to warm up and stabilize.
- Lab temperature fluctuation can affect the baseline stability.
- System back pressure must be monitored as it can damage the flow cell.
- Waste tubing must be in right position as per detector.
- Some times sticky or highly concentrated sample can contaminate the flow cell, pass suitable solvent through flow cell.
Column Care
- Refer the column manual for their optimum use and care.
- Washing procedure must be w.r.t. column type (C-18 is hydrophobic column and needs high organic wash which may not work for polar –NH2 column)
- Generally 5-10 column volume of water must be passed to remove the salts and finally 15-20 column volume of organic solvent (At lease 95%).
- Use guard column for better column life.
- Ferrules selection can affect the peak shape.
- Mobile phase must be filtered to give longer column life.
Track on column back pressure helps in optimizing the washing procedure.
Troubleshooting- RT and area variation and area variation- The possible causes may be
*Mobile phase composition changes *Poorly miscible mobile phase *Insufficient equilibration or bad column *Change in temperature (TCC) *Improper Injector setting *Vial type & filling *Detector equilibration *Change in column condition *Diluent or sample nature *Carry over Let’s avoid Overfilling the vial *Avoid inverting the vial *Over tightening the vial cap
The base line drifting may be due to
- Temperature unstable (Refractive Index Detector)
- Gradient elution, need to verify the gradient elution.
- Contamination in Mobile Phase may cause big issue.
- Mobile phase not in equilibrium with column and needs time.
- Column bleeding and time to change.
